scotty
Member
Chicago Fish and Coral Company have a blog on their website. Parts omitted are the sale of testing in their store, if you wish to have an unabridged version, it is on:
http://www.chicagofishandcoral.com/blog/
They put together a three part analysis of water chemistry that I will share below because I think it's a hugely informative piece, and will help when we have people later on asking the same questions over and over, we have something to point them to, I did the leg work, now they gotta read.
Water Quality pt. 1
7th Jun 2010 | Posted in: Water Quality 0
We will spend the next few weeks looking at ways to improve the most important component of all our aquariums…WATER. Each week we will touch on an aspect of water quality, and particularly how some of these pertain to summertime aquarium maintenance. We will also be putting on sale the products we will be talking about.
Summertime and your fish tank
Summer is now upon us, and as it heats up outside it’s easy to spend less time attending to our tanks than in the cooler months. The killer B’s – Baseball, Barbecues and Backyards – take a good deal of time away from your fish tank. Water changes seem to become less frequent, maybe the fish don’t always get fed on time. Also the warmer temperatures can bring problems to reef tanks without chillers as water temperatures can rise dramatically.
So first off, when was the last time you tested your water?
I admittedly had not done a test on my own tank for quite a long time. The other day I went ahead and did a complete test and discovered that I had a phosphate problem. Even with using a refugium and RO water for both water changes and top-off, phosphate can build up in a tank over time. Remember that what we feed our tanks becomes organic waste matter, whether its inhabitants eat it or not, and is a large source of nutrients such as phosphate. Rather than a large water change, I added some chemical products to combat the problem. A week later corals responded with more polyp extension and algae was not growing as quickly on the front glass.
While chemical products such as phosphate/nitrate removers or other similar absorbers are not substitutes for good husbandry, they can be useful in a pinch, and may prolong the time needed between water changes.
The standard water test includes:
PH
NITRATE / NITRITE
AMMONIA
PHOSPHATE
CALCIUM
ALKALINITY
Water Quality pt. 2 - Ph
22nd Jun 2010 | Posted in: Water Quality 1
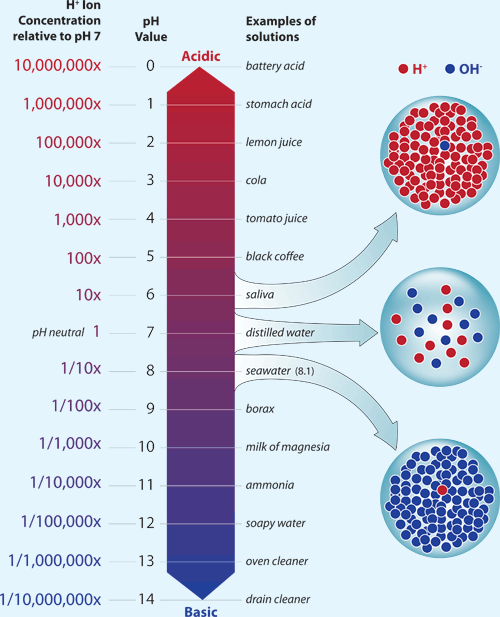
pH, it goes up, and it goes down. Sometimes it can change over the course of a day, other times over months. Our fish,of course, would prefer that it doesn’t change at all. So why does it change, and what can we do about it?
pH Basics (and Acids)
Loosely defined, pH is a measure of Hydrogen ions in the water, and the lower the pH, the more acidic the water is. The scale ranges from 0-14, but in a saltwater tank we’re focused on a small portion of this range, between 7.6 and 8.4. This may not seem like much, but the pH scale is logarithmic (much like the Richter scale), meaning a pH of 7.6 is almost ten times more acidic than a pH of 8.4!
A fish-only system can actually tolerate a pH anywhere in this range, provided it is stable, and the fish get accustomed to it slowly. This is a major reason why we have to acclimate fish (and invertebrates), as even a small change in pH can be quite a shock. Reef systems are a different story, as their inhabitants require a narrower pH range, between 8.0 and 8.4, and are less tolerant of pH changes.
The task, then, of controlling pH can seem difficult, and it is complicated by the fact that aquariums have a tendency to become more and more acidic over time. This is because acids are constantly being added to the water, in the form of CO2 from respiration, nitrification by way of biological filtration, and organics from foods and biological waste. These acids lower the overall pH of the water, sometimes drastically.
Luckily, both marine and freshwater systems have a means of naturally combating this acid attack. Alkalinity (aka carbonate hardness, KH, or dKH) is a measure of carbonates, which provide a buffer that can slow changes to the pH of the water. In reef systems, these same carbonates are utilized by corals to build their skeletons and can diminish overtime, so it is especially important to keep a close eye on alkalinity and pH. To be brief, you should aim for an alkalinity between 7-11 dKH depending on the system. The higher the alkalinity, the more buffering capacity, and the more acids the system can handle. If the level of carbonates becomes too high however, it can disrupt the ability of other compounds to remain dissolved in the water, throwing off the overall balance.
In both fish-only and reef aquariums, when the pH starts to drop, it is usually an indication that the buffering capacity of the water is getting worn out, and the increase in acidity needs to be corrected.
Ways to Stabilize and Solve pH Problems
Change your water! – This common-sense tactic is our favorite method of raising and/or stabilizing pH and dKH levels, because regular partial water changes refresh the natural carbonate buffers and restore other major elements in the water column. Remember, what you put into your tank in the form of fish (respiration) and foods (organic waste) must be removed or neutralized so you can maintain a high pH and clean environment, and this is the easiest way to accomplish it.
The Quick Fix – There are many good pH stabilizers and adjusting products on the market that are suitable for a wide range of situations. These products come in either powder or liquid form and many can be added by dosing on a regular basis without raising the pH above 8.4. It should be noted, though, that some of these products raise alkalinity as well as pH. Since excessive usage can raise both of these to dangerously high levels, it’s important to test both alkalinity and pH periodically when following a dosing regimen.
Even if you are not currently on a dosing regimen or have a pH problem, it is a good idea to have a pH stabilizer on-hand, as you will never know if/when you will need it.
Calcium Reactors/Automatic Dosers – These are expensive solutions to control pH and alkalinity. Reef geeks with larger systems love these contraptions because they provide a consistent environment to their vast array of corals. While these systems do work, for many hobbyists they are simply an added expense and liability, so we only recommend them for larger systems with a high coral population.
Testing and pH Buffer Specials
Once again start with the basics. If you are not testing your pH, you don’t know if it is a problem.
http://www.chicagofishandcoral.com/blog/
They put together a three part analysis of water chemistry that I will share below because I think it's a hugely informative piece, and will help when we have people later on asking the same questions over and over, we have something to point them to, I did the leg work, now they gotta read.
Water Quality pt. 1
7th Jun 2010 | Posted in: Water Quality 0
We will spend the next few weeks looking at ways to improve the most important component of all our aquariums…WATER. Each week we will touch on an aspect of water quality, and particularly how some of these pertain to summertime aquarium maintenance. We will also be putting on sale the products we will be talking about.
Summertime and your fish tank
Summer is now upon us, and as it heats up outside it’s easy to spend less time attending to our tanks than in the cooler months. The killer B’s – Baseball, Barbecues and Backyards – take a good deal of time away from your fish tank. Water changes seem to become less frequent, maybe the fish don’t always get fed on time. Also the warmer temperatures can bring problems to reef tanks without chillers as water temperatures can rise dramatically.
So first off, when was the last time you tested your water?
I admittedly had not done a test on my own tank for quite a long time. The other day I went ahead and did a complete test and discovered that I had a phosphate problem. Even with using a refugium and RO water for both water changes and top-off, phosphate can build up in a tank over time. Remember that what we feed our tanks becomes organic waste matter, whether its inhabitants eat it or not, and is a large source of nutrients such as phosphate. Rather than a large water change, I added some chemical products to combat the problem. A week later corals responded with more polyp extension and algae was not growing as quickly on the front glass.
While chemical products such as phosphate/nitrate removers or other similar absorbers are not substitutes for good husbandry, they can be useful in a pinch, and may prolong the time needed between water changes.
The standard water test includes:
PH
NITRATE / NITRITE
AMMONIA
PHOSPHATE
CALCIUM
ALKALINITY
Water Quality pt. 2 - Ph
22nd Jun 2010 | Posted in: Water Quality 1

pH, it goes up, and it goes down. Sometimes it can change over the course of a day, other times over months. Our fish,of course, would prefer that it doesn’t change at all. So why does it change, and what can we do about it?
pH Basics (and Acids)
Loosely defined, pH is a measure of Hydrogen ions in the water, and the lower the pH, the more acidic the water is. The scale ranges from 0-14, but in a saltwater tank we’re focused on a small portion of this range, between 7.6 and 8.4. This may not seem like much, but the pH scale is logarithmic (much like the Richter scale), meaning a pH of 7.6 is almost ten times more acidic than a pH of 8.4!
A fish-only system can actually tolerate a pH anywhere in this range, provided it is stable, and the fish get accustomed to it slowly. This is a major reason why we have to acclimate fish (and invertebrates), as even a small change in pH can be quite a shock. Reef systems are a different story, as their inhabitants require a narrower pH range, between 8.0 and 8.4, and are less tolerant of pH changes.
The task, then, of controlling pH can seem difficult, and it is complicated by the fact that aquariums have a tendency to become more and more acidic over time. This is because acids are constantly being added to the water, in the form of CO2 from respiration, nitrification by way of biological filtration, and organics from foods and biological waste. These acids lower the overall pH of the water, sometimes drastically.
Luckily, both marine and freshwater systems have a means of naturally combating this acid attack. Alkalinity (aka carbonate hardness, KH, or dKH) is a measure of carbonates, which provide a buffer that can slow changes to the pH of the water. In reef systems, these same carbonates are utilized by corals to build their skeletons and can diminish overtime, so it is especially important to keep a close eye on alkalinity and pH. To be brief, you should aim for an alkalinity between 7-11 dKH depending on the system. The higher the alkalinity, the more buffering capacity, and the more acids the system can handle. If the level of carbonates becomes too high however, it can disrupt the ability of other compounds to remain dissolved in the water, throwing off the overall balance.
In both fish-only and reef aquariums, when the pH starts to drop, it is usually an indication that the buffering capacity of the water is getting worn out, and the increase in acidity needs to be corrected.
Ways to Stabilize and Solve pH Problems
Change your water! – This common-sense tactic is our favorite method of raising and/or stabilizing pH and dKH levels, because regular partial water changes refresh the natural carbonate buffers and restore other major elements in the water column. Remember, what you put into your tank in the form of fish (respiration) and foods (organic waste) must be removed or neutralized so you can maintain a high pH and clean environment, and this is the easiest way to accomplish it.
The Quick Fix – There are many good pH stabilizers and adjusting products on the market that are suitable for a wide range of situations. These products come in either powder or liquid form and many can be added by dosing on a regular basis without raising the pH above 8.4. It should be noted, though, that some of these products raise alkalinity as well as pH. Since excessive usage can raise both of these to dangerously high levels, it’s important to test both alkalinity and pH periodically when following a dosing regimen.
Even if you are not currently on a dosing regimen or have a pH problem, it is a good idea to have a pH stabilizer on-hand, as you will never know if/when you will need it.
Calcium Reactors/Automatic Dosers – These are expensive solutions to control pH and alkalinity. Reef geeks with larger systems love these contraptions because they provide a consistent environment to their vast array of corals. While these systems do work, for many hobbyists they are simply an added expense and liability, so we only recommend them for larger systems with a high coral population.
Testing and pH Buffer Specials
Once again start with the basics. If you are not testing your pH, you don’t know if it is a problem.

